Akeso's Disappointing Trial Results Send Shares Plummeting

Table of Contents
The Clinical Trial in Question: Specifics and Methodology
The significant drop in Akeso's stock price directly correlates with the results released from its Phase III clinical trial evaluating [Insert Drug Name], a [Insert Drug Type] intended for the treatment of [Insert Target Indication]. This large-scale trial, involving [Number] patients across [Number] global sites, aimed to demonstrate the superiority of [Insert Drug Name] compared to the current standard of care. The primary endpoint of the trial was [State Primary Endpoint, e.g., progression-free survival].
- Trial Design: A randomized, double-blind, placebo-controlled study.
- Patient Population: Patients with [Specific Patient Characteristics, e.g., advanced stage disease, specific genetic markers].
- Methodology: [Describe the methods used to collect and analyze data, e.g., imaging techniques, blood tests, patient-reported outcomes].
- Key Akeso clinical trial inclusion criteria: [List Key inclusion and exclusion criteria].
The meticulous design of this Akeso clinical trial initially instilled confidence, making the subsequent disappointing results all the more impactful.
Analysis of the Disappointing Results: What Went Wrong?
The released data revealed that the trial failed to meet its primary endpoint. [Insert Drug Name] did not demonstrate statistically significant improvement in [Primary Endpoint] compared to the placebo group. While the exact data will require further analysis, initial reports suggest [Explain the reasons for failure, e.g., lower-than-expected response rates, higher-than-expected adverse events]. The lack of statistical significance in the primary endpoint raises significant concerns about the drug's efficacy in the target patient population.
- Key Findings:
- Non-significant improvement in the primary endpoint.
- [Mention specific numerical data, e.g., Hazard Ratio of X, p-value of Y].
- [Mention any significant adverse events or safety concerns].
The absence of statistically significant efficacy data, coupled with [mention safety concerns if applicable], is the primary reason behind Akeso's disappointing trial results and the subsequent market reaction.
Market Reaction and Investor Sentiment: Akeso Stock Plummets
News of Akeso's disappointing trial results triggered an immediate and significant sell-off. Akeso's stock price plummeted by [Percentage]% within [Timeframe] of the announcement. Market capitalization decreased drastically, wiping out [Dollar Amount] in shareholder value. Analyst commentary has been predominantly negative, with several firms downgrading their ratings and price targets for Akeso stock. Investor confidence has clearly eroded, reflecting the gravity of the setback for the company.
- Stock Price Impact: [Specific data points on stock price changes].
- Trading Volume: [Data on increased trading volume following the announcement].
- Analyst Ratings: [Summary of analyst reactions and revised ratings].
The rapid and severe market reaction underscores the high expectations placed on [Insert Drug Name] and the significant disappointment resulting from the failed trial.
Future Outlook and Potential Implications for Akeso
The long-term implications of these disappointing trial results remain uncertain. Akeso's future prospects depend on several factors, including the company's ability to analyze the trial data thoroughly, identify any potential contributing factors to the failure, and adjust its clinical development strategy accordingly. Akeso might need to reassess its drug development approach or consider alternative indications for [Insert Drug Name]. The company's overall pipeline and the success of its other research programs will also play a critical role in determining its future trajectory.
- Future Clinical Trials: [Mention any planned future trials or adjustments to the development plan].
- Regulatory Pathways: [Discuss potential changes to regulatory pathways and timelines].
- Financial Implications: [Discuss the potential financial impact, e.g., reduced funding, potential layoffs].
The future of Akeso hangs in the balance, dependent on its response to Akeso's disappointing trial results and its ability to navigate this significant hurdle.
Conclusion: Understanding the Impact of Akeso's Disappointing Trial Results
Akeso's disappointing trial results have sent shockwaves through the market, causing a significant drop in its share price and raising serious concerns about the future of its lead drug candidate. The failure to meet the primary endpoint highlights the inherent risks in drug development and the importance of rigorous clinical trial design and execution. Understanding the full scope of Akeso's disappointing trial results is crucial for investors and stakeholders alike. Stay updated on the latest developments in Akeso's clinical trials and the evolving impact on its stock price by following [link to relevant source, e.g., company website, financial news website].

Featured Posts
-
 Rise In Adhd Cases Among Young People At Aiims Opd Exploring Potential Triggers
Apr 29, 2025
Rise In Adhd Cases Among Young People At Aiims Opd Exploring Potential Triggers
Apr 29, 2025 -
 2025 Porsche Cayenne Picture Gallery Exterior And Interior Designs
Apr 29, 2025
2025 Porsche Cayenne Picture Gallery Exterior And Interior Designs
Apr 29, 2025 -
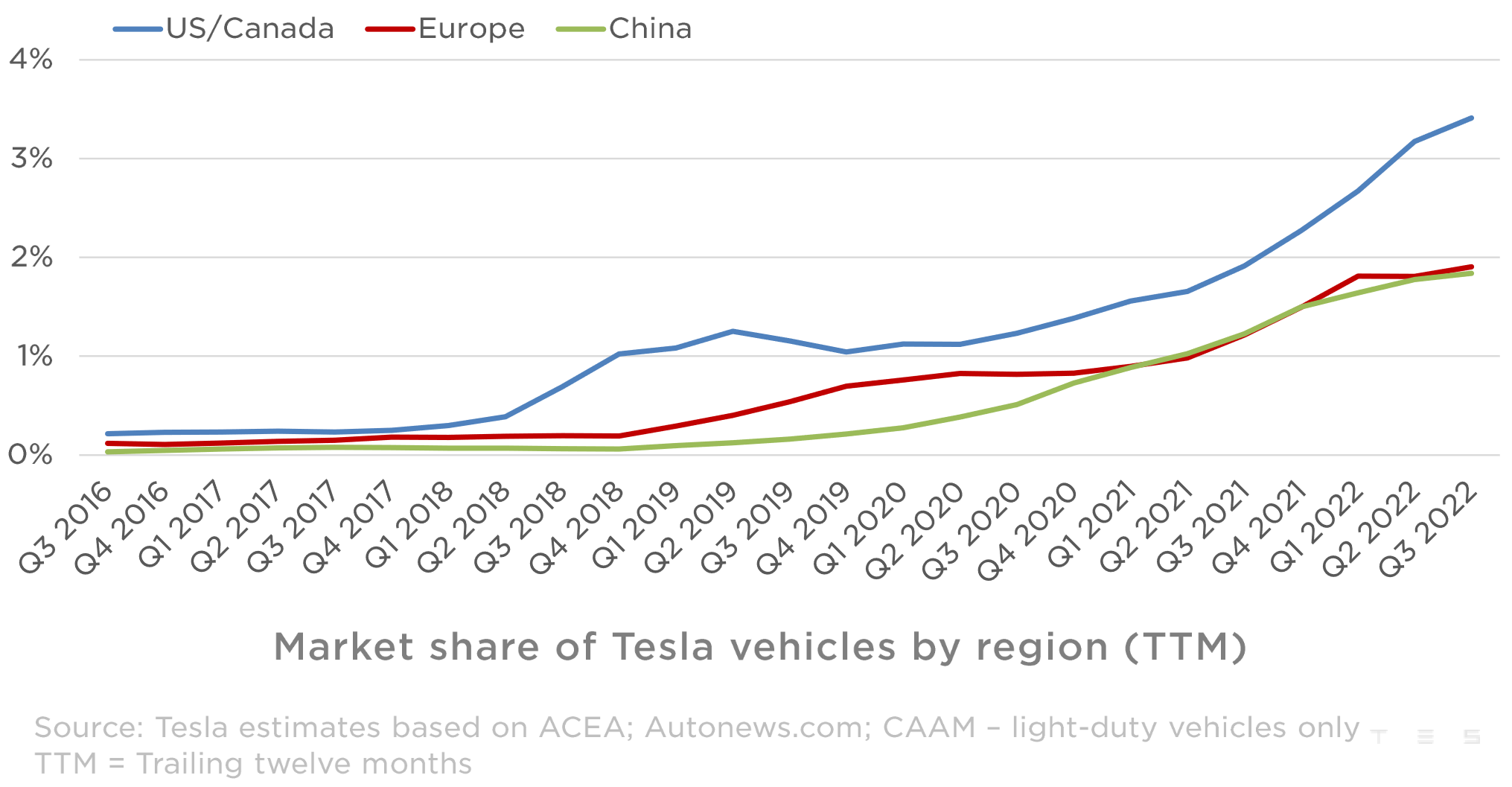 Chinas Impact On Bmw And Porsche Sales Market Share And Future Outlook
Apr 29, 2025
Chinas Impact On Bmw And Porsche Sales Market Share And Future Outlook
Apr 29, 2025 -
 Capital Summertime Ball 2025 Tickets Your Guide To Securing Entry
Apr 29, 2025
Capital Summertime Ball 2025 Tickets Your Guide To Securing Entry
Apr 29, 2025 -
 Ukraine War North Koreas Admission Of Troop Deployment To Russia
Apr 29, 2025
Ukraine War North Koreas Admission Of Troop Deployment To Russia
Apr 29, 2025
