Analysis: How Trump's FDA Shaped The Biotech Landscape

Table of Contents
Accelerated Drug Approvals under Trump's FDA
The Trump administration prioritized faster drug approvals, aiming to expedite access to potentially life-saving medications. This push manifested in several key areas.
Right-to-Try Initiatives and their Impact
The "Right to Try" movement gained momentum during this period, allowing terminally ill patients access to experimental drugs not yet approved by the FDA.
- Increased patient access: Right-to-Try provided a potential avenue for patients with limited treatment options.
- Ethical considerations: The initiative sparked debates regarding informed consent, clinical trial integrity, and the potential for exploitation of vulnerable patients.
- Impact on clinical trials: Some argued that Right-to-Try could compromise the integrity of ongoing clinical trials by impacting patient recruitment and data reliability.
- Potential risks and benefits: While offering hope, the initiative also raised concerns about unproven treatments and the potential for adverse effects without adequate monitoring. The balance between expedited access and patient safety remained a critical discussion point. The impact of Right to Try on the FDA approval process itself was minimal, as it did not alter formal approval pathways.
Changes to the Drug Approval Process
The Trump administration implemented changes designed to streamline the FDA's drug approval pathways.
- Faster review times: The FDA prioritized review programs, aiming to reduce the time it took to approve new drugs. This impacted the timelines for various phases of the drug approval process.
- Impact on drug pricing: Accelerated approvals might indirectly influence drug pricing by potentially bringing medications to market sooner, but also raised concerns about whether this speed compromised price negotiation leverage.
- Influence on pharmaceutical innovation: The faster approval process could potentially incentivize pharmaceutical innovation by offering quicker returns on investment, although critics argued that it might also incentivize shortcuts in the research and development process. The long-term impact of changes in FDA regulations on innovation is complex and still being studied.
Impact on Biotech Funding and Investment
The changes implemented under Trump's FDA had a significant impact on the biotech investment landscape.
Shift in Investor Sentiment
The regulatory changes influenced investor confidence in the biotech sector.
- Increased or decreased investment: Depending on the specific area of biotech and investor risk tolerance, the changes may have resulted in either increased or decreased investments. Some investors saw opportunities in faster approvals, while others were concerned about increased regulatory uncertainty.
- Impact on venture capital: Venture capital firms adjusted their investment strategies based on perceived risks and opportunities stemming from the altered FDA regulatory environment.
- Mergers and acquisitions activity: The modified regulatory climate could have affected the frequency and nature of mergers and acquisitions within the biotech industry.
Effects on Biotech Company Stock Prices
FDA policies and their interpretations had a direct correlation with fluctuations in biotech stock prices.
- Case studies of specific companies: Examining individual company performance during this period provides valuable insights into how different firms responded to regulatory changes.
- Analysis of stock performance data: Analyzing stock performance data alongside FDA decisions allows for a better understanding of market reactions to specific policy shifts. This requires a comprehensive analysis of market sentiment.
- Long-term effects on market capitalization: The long-term effects of the Trump administration's policies on the overall market capitalization of biotech companies are still being assessed.
Controversies and Criticisms of Trump's FDA Approach
Despite the focus on faster approvals, Trump's FDA approach faced significant criticism.
Concerns about Safety and Efficacy
Critics raised concerns that the emphasis on speed might compromise drug safety and efficacy.
- Examples of controversial drug approvals: Specific examples of drug approvals during this period can be analyzed to illustrate potential concerns about safety and efficacy trade-offs.
- Public health concerns: Concerns about potential adverse events or long-term health consequences linked to potentially hastily approved medications were voiced by various stakeholders.
- Potential long-term consequences: The long-term effects of expedited approvals on public health are a matter of ongoing investigation and debate.
Political Influence on Regulatory Decisions
Allegations of political influence on FDA decision-making processes arose during this period.
- Specific examples of alleged political interference: Documented instances of perceived political influence need to be critically examined.
- Impact on public trust in the FDA: Accusations of political interference eroded public trust in the FDA's independence and impartiality, which is critical to the successful functioning of the regulatory process.
Conclusion
The Trump administration's influence on the FDA significantly reshaped the biotech landscape, leading to both accelerated drug approvals and considerable controversy. While faster approval processes potentially boosted innovation and patient access, concerns remain regarding potential compromises on safety and efficacy, as well as the perception of political influence on regulatory decisions. The long-term implications of these changes continue to unfold and require ongoing monitoring.
Call to Action: Further analysis of Trump's FDA and its lasting impact on the biotech industry is crucial. Understanding the intricacies of these regulatory shifts is essential for navigating the ever-evolving landscape of pharmaceutical development and investment. Continue exploring the effects of Trump's FDA on the biotech landscape to gain a comprehensive understanding of its legacy.

Featured Posts
-
 Cy Young Winners Dominant April Performance 9 Run Lead Strikeout Fury
Apr 23, 2025
Cy Young Winners Dominant April Performance 9 Run Lead Strikeout Fury
Apr 23, 2025 -
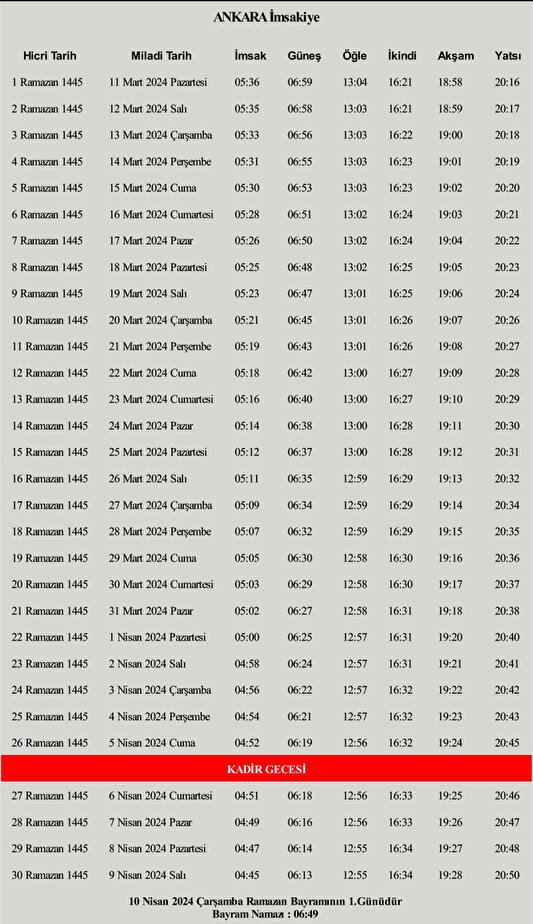 3 Mart 2024 Pazartesi Ankara Iftar Ve Sahur Vakti
Apr 23, 2025
3 Mart 2024 Pazartesi Ankara Iftar Ve Sahur Vakti
Apr 23, 2025 -
 La Carte Blanche Comprendre L Approche De Marc Fiorentino
Apr 23, 2025
La Carte Blanche Comprendre L Approche De Marc Fiorentino
Apr 23, 2025 -
 Son Dakika Erzurum Okullari Tatil Mi Degil Mi 24 Subat Bilgileri
Apr 23, 2025
Son Dakika Erzurum Okullari Tatil Mi Degil Mi 24 Subat Bilgileri
Apr 23, 2025 -
 Amandine Gerard Je T Aime Moi Non Plus Analyse Des Relations Commerciales Euro Marches
Apr 23, 2025
Amandine Gerard Je T Aime Moi Non Plus Analyse Des Relations Commerciales Euro Marches
Apr 23, 2025
