Biotech Industry Receives Boost From Trump-Era FDA Policies
Table of Contents
Accelerated Drug Approvals under Operation Warp Speed
Operation Warp Speed, the Trump administration's initiative to accelerate the development and distribution of COVID-19 vaccines and therapeutics, stands as a prime example of the impact of altered FDA policies on the biotech industry. This program dramatically reshaped the drug approval process, prioritizing speed without compromising safety. The initiative's success hinged on streamlining regulatory pathways, leading to a significant reduction in drug development timelines.
- Examples of drugs/vaccines approved under accelerated pathways: The Pfizer-BioNTech and Moderna COVID-19 vaccines, along with several antiviral treatments like remdesivir, were developed and approved at an unprecedented pace.
- Specific changes in FDA procedures that led to faster approvals: This included the increased use of emergency use authorizations (EUAs), parallel testing and manufacturing, and the prioritization of applications for COVID-19 related products. The FDA also embraced rolling reviews, allowing companies to submit data as it became available, rather than waiting for completion of all trials.
- Impact on the speed of biotech innovation: Operation Warp Speed showcased the potential for significantly accelerated drug development, inspiring innovation and investment in other areas of biotechnology. The experience gained from this initiative provided valuable insights that could be applied to future drug development efforts.
Reduced Regulatory Burden and Streamlined Processes
Beyond Operation Warp Speed, the Trump administration implemented several policies aimed at reducing the regulatory burden on biotech companies. These changes were intended to foster a more efficient drug development process and encourage greater investment in the sector.
- Examples of specific regulations that were modified or relaxed: While not all changes were explicitly stated as being part of a singular policy, the overall effect was a more streamlined review process. This included adjustments to the requirements for clinical trials, data submission, and post-market surveillance, which was perceived by many as a welcome change after years of relatively slower approvals.
- The positive impact on biotech company investment and growth: The perception of a less burdensome regulatory environment attracted significant investment. Venture capital flowed into the biotech sector, fueling research and development, and boosting the market capitalization of many established companies.
- Data supporting the claim of reduced regulatory burden: Although quantifying the precise reduction in regulatory burden is challenging, analyzing the approval times for drugs during this period compared to previous years provides some evidence of accelerated processes. Furthermore, an increase in the number of new biotech startups during this period also suggests a more favorable regulatory climate.
Impact on Biotech Investment and Growth
The changes in FDA policies under the Trump administration correlated with a notable increase in biotech investment and growth. This surge in funding and activity helped propel the industry forward, leading to more innovation and increased access to new therapies.
- Statistics on venture capital investment in biotech during the relevant period: Data from sources like PitchBook and the National Venture Capital Association show a significant rise in venture capital investment in the biotech sector during the Trump administration.
- Changes in the market capitalization of major biotech companies: Many major biotech companies experienced significant increases in their market capitalization during this period, reflecting investor confidence in the sector's future.
- Number of new biotech startups established during this time: The number of new biotech startups founded during this era indicates a thriving environment for entrepreneurial activity and innovation within the sector.
Potential Drawbacks and Future Considerations
While the accelerated approvals and reduced regulatory burden under the Trump administration led to significant advancements in the biotech industry, it's crucial to acknowledge potential drawbacks. The emphasis on speed raised concerns about the thoroughness of safety evaluations.
- Discussion of potential safety concerns associated with expedited approvals: Some argued that the accelerated approval process might have compromised the rigorous assessment of long-term safety risks associated with certain drugs.
- The importance of robust post-market surveillance: To mitigate potential risks, enhanced post-market surveillance is crucial to monitor for any adverse effects of drugs approved through expedited pathways.
- The need for a careful balancing act between speed and safety in drug development: Finding the right equilibrium between expediting the approval process and maintaining stringent safety standards remains a critical challenge for the FDA and the biotech industry.
Conclusion
In conclusion, the Trump administration's policies significantly impacted the biotech industry, resulting in faster drug approvals, a reduced regulatory burden, and increased investment and growth. Operation Warp Speed stands out as a prominent example of the potential benefits of streamlined regulation, while concerns about potential safety compromises highlight the ongoing need for careful consideration. The long-term effects of these policies on patient access to new treatments and the overall health of the biotech industry are still unfolding and warrant continued investigation. To better understand the complexities of biotech regulation and its future, further research into specific FDA policies from the Trump administration is essential. Continue exploring the impact of FDA policies on the biotech industry to gain a deeper understanding of this dynamic field.

Featured Posts
-
 Yelich Returns First Spring Start After Back Surgery
Apr 23, 2025
Yelich Returns First Spring Start After Back Surgery
Apr 23, 2025 -
 Le Sans Alcool Opportunites Economiques Et Benefices Sante Avec La Tournee Minerale Et Dry January
Apr 23, 2025
Le Sans Alcool Opportunites Economiques Et Benefices Sante Avec La Tournee Minerale Et Dry January
Apr 23, 2025 -
 New York Yankees Broadcasters Controversial Seattle Mariners Comment
Apr 23, 2025
New York Yankees Broadcasters Controversial Seattle Mariners Comment
Apr 23, 2025 -
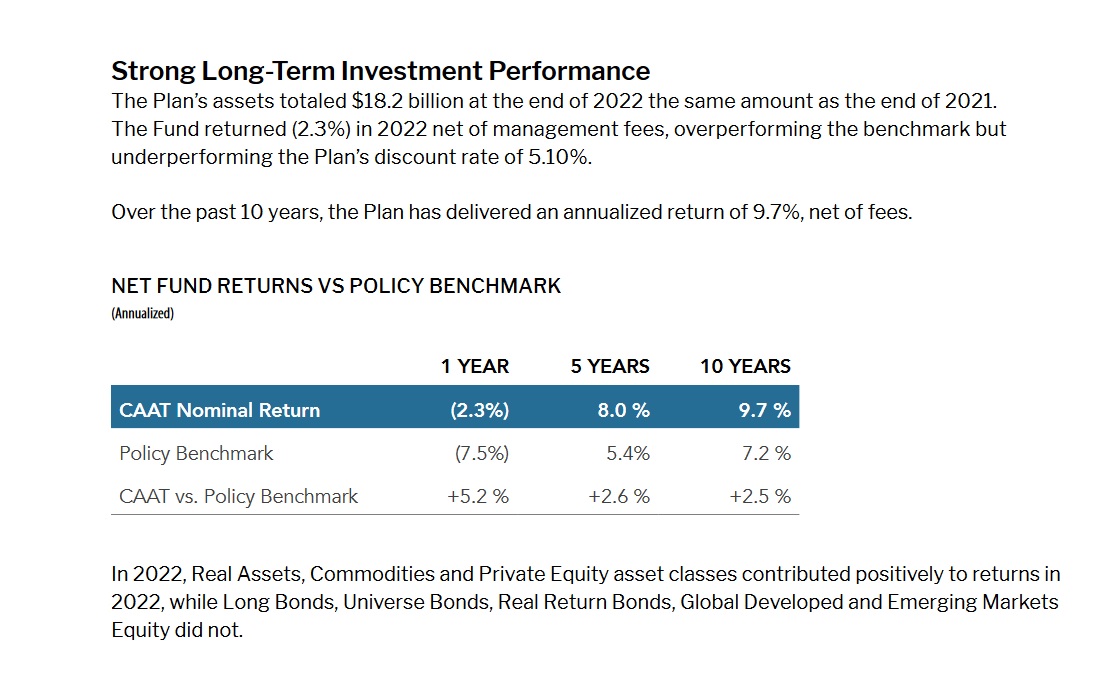 Caat Pension Plan Expands Search For Canadian Private Investments
Apr 23, 2025
Caat Pension Plan Expands Search For Canadian Private Investments
Apr 23, 2025 -
 Pavel Pivovarov Anonsiroval Sovmestniy Merch S Aleksandrom Ovechkinym
Apr 23, 2025
Pavel Pivovarov Anonsiroval Sovmestniy Merch S Aleksandrom Ovechkinym
Apr 23, 2025
