Powerful CRISPR: Precise Whole Gene Insertion Into Human DNA
Table of Contents
Understanding the Mechanics of CRISPR-Cas9 for Whole Gene Insertion
CRISPR-Cas9, a game-changing gene editing technology, acts like a highly precise pair of molecular scissors. It utilizes a guide RNA (gRNA) molecule, designed to match a specific DNA sequence within the genome, to direct the Cas9 enzyme to that precise location. The Cas9 enzyme then creates a double-stranded break in the DNA. For whole gene insertion, the cell’s natural repair mechanism, homology-directed repair (HDR), is leveraged.
HDR uses a provided DNA template – the donor DNA containing the desired gene – to repair the break. This process accurately inserts the whole gene into the targeted location.
The process involves several key steps:
- Designing the gRNA: A gRNA molecule is designed to be complementary to the target DNA sequence where the gene needs to be inserted.
- Delivery of CRISPR components: The gRNA and Cas9 enzyme are delivered into the target cells (e.g., using viral vectors or electroporation).
- Creating the double-stranded break: The gRNA guides the Cas9 enzyme to the target site, creating a precise cut in the DNA.
- HDR-mediated repair: The cell's natural HDR pathway uses the provided donor DNA template containing the whole gene to accurately repair the break, integrating the new gene into the genome.
- Verification of insertion: Techniques like PCR and sequencing are used to confirm the successful and precise insertion of the whole gene.
(Illustrative diagram would be placed here)
Challenges and Limitations of Precise Whole Gene Insertion
While powerful, CRISPR-Cas9 technology for whole gene insertion faces several challenges:
- Low HDR efficiency: HDR is often less efficient than other DNA repair pathways, leading to a low rate of successful gene insertion. Non-homologous end joining (NHEJ), another DNA repair pathway, can often dominate, resulting in undesired mutations.
- Off-target effects: The CRISPR-Cas9 system can sometimes cut DNA at unintended locations (off-target sites), causing potentially harmful mutations. Minimizing these off-target effects is crucial.
- Delivery challenges: Efficient and safe delivery of the CRISPR-Cas9 system and donor DNA into the target cells remains a significant hurdle. Methods like viral vectors can trigger immune responses, while non-viral methods often have lower delivery efficiency.
The major hurdles summarized:
- Inefficient Homology-Directed Repair (HDR)
- Prevalence of Off-Target Cuts
- Challenges in Effective Delivery Systems
Advancements and Innovations in CRISPR-Cas9 Technology for Whole Gene Insertion
Significant progress is being made to overcome the limitations of CRISPR-Cas9 technology:
- High-fidelity Cas9 variants: Engineered Cas9 variants, such as SpCas9-HF1 and eSpCas9(1.1), exhibit significantly reduced off-target activity, increasing the precision of gene editing.
- Advanced delivery methods: Researchers are exploring innovative delivery methods, including improved viral vectors with reduced immunogenicity and advanced non-viral techniques like lipid nanoparticles and electroporation.
- Strategies to enhance HDR efficiency: Techniques such as using DNA repair inhibitors to suppress NHEJ and promote HDR are being actively investigated. Furthermore, the use of ssODNs (single-stranded oligodeoxynucleotides) as donor templates is gaining traction due to its higher efficiency in HDR.
Key advancements include:
- Development of High-Fidelity Cas9 Nucleases
- Improved Viral and Non-Viral Delivery Systems
- Strategies to Enhance HDR Efficiency
Potential Applications of Precise Whole Gene Insertion in Human Therapeutics
Precise whole gene insertion holds immense potential across various therapeutic areas:
- Gene therapy: Correcting genetic defects by inserting functional copies of mutated genes responsible for inherited diseases like cystic fibrosis and sickle cell anemia.
- Cancer immunotherapy: Modifying immune cells (e.g., T cells) to enhance their ability to target and destroy cancer cells. This involves inserting genes that encode for chimeric antigen receptors (CARs).
- Regenerative medicine: Correcting genetic defects in stem cells to generate healthy tissues and organs for transplantation.
Potential therapeutic uses:
- Treatment of Genetic Disorders
- Enhancement of Cancer Immunotherapy
- Advancements in Regenerative Medicine
Ethical considerations are paramount. The potential for unintended consequences necessitates rigorous safety testing and careful ethical review before clinical applications. The long-term effects of gene editing need thorough investigation.
Conclusion: The Future of Powerful CRISPR Gene Editing
The advancements in powerful CRISPR technology for precise whole gene insertion are transforming the landscape of genetic engineering. While challenges remain, ongoing research is continuously improving the efficiency, specificity, and safety of this revolutionary technology. The potential applications in treating a wide range of diseases, from genetic disorders to cancer, are immense.
The future of medicine is intertwined with the advancements in powerful CRISPR technology. Continue to explore the latest research and engage in the ethical dialogue surrounding this groundbreaking field. Learn more about CRISPR-Cas9, its potential applications, and the ongoing ethical considerations to become a more informed participant in shaping its future.

Featured Posts
-
 Covid 19 Pandemic Lab Owners Guilty Plea For False Test Results
May 30, 2025
Covid 19 Pandemic Lab Owners Guilty Plea For False Test Results
May 30, 2025 -
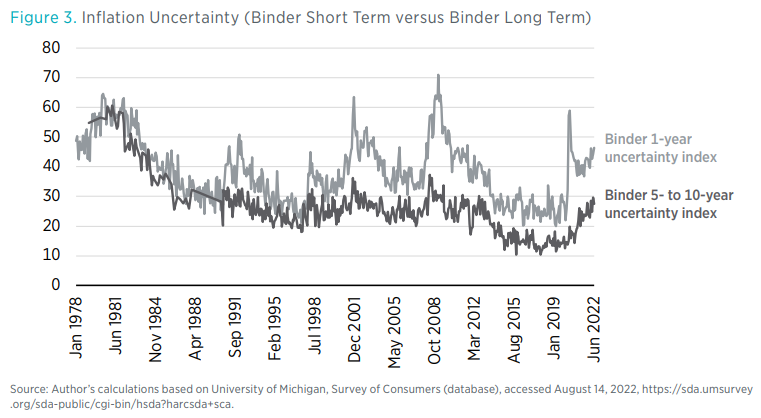 The Impact Of Rising Inflation And Unemployment On Economic Uncertainty
May 30, 2025
The Impact Of Rising Inflation And Unemployment On Economic Uncertainty
May 30, 2025 -
 Badetemperaturer Og Vaer En Praktisk Guide For Sommeren
May 30, 2025
Badetemperaturer Og Vaer En Praktisk Guide For Sommeren
May 30, 2025 -
 Ticketmaster Y Setlist Fm La Guia Definitiva Para Fans De Conciertos
May 30, 2025
Ticketmaster Y Setlist Fm La Guia Definitiva Para Fans De Conciertos
May 30, 2025 -
 Tennis Governance Under Fire Djokovics Union Launches Legal Action
May 30, 2025
Tennis Governance Under Fire Djokovics Union Launches Legal Action
May 30, 2025
