Trump's FDA And Its Impact On The Biotech Industry's Future
Table of Contents
Accelerated Drug Approvals Under Trump's FDA:
The Trump administration championed accelerated drug approvals, aiming to expedite access to potentially life-saving treatments. This approach, while lauded by some, sparked considerable debate within the scientific community.
-
Projecting the impact of Right-to-Try initiatives: The "Right to Try" initiative, allowing terminally ill patients access to experimental drugs outside of clinical trials, further accelerated the pace of drug access. While seemingly compassionate, this initiative raised concerns about the lack of robust data on efficacy and safety. The long-term impact on the development of effective therapies remains to be seen.
-
The impact on clinical trial speed and regulatory burden: Efforts to streamline the regulatory pathway resulted in some improvements in the speed of clinical trials. However, the long-term effects on the rigor and thoroughness of testing remain a subject of ongoing discussion. Data from this period is still being analyzed to fully understand its effects. The reduced regulatory burden, while aiming to improve efficiency, also potentially introduced risks.
- Reduced review times for certain applications.
- Increased use of breakthrough therapy designations.
- Potential for overlooking critical safety concerns.
Changes in FDA Funding and Resource Allocation:
Changes in FDA funding and resource allocation during the Trump administration significantly impacted its ability to oversee the biotech industry.
-
Impact of budget cuts or increases on drug development and review processes: While specific budget changes varied year to year, overall funding levels and their allocation to different divisions within the FDA influenced the speed and quality of drug reviews. Reductions in certain areas potentially led to longer review times or less rigorous scrutiny.
-
Influence on regulatory oversight and enforcement: Budgetary constraints could have impacted the FDA's ability to effectively monitor the safety and efficacy of approved drugs post-market. This raises concerns about potential long-term public health implications.
- Potential delays in inspections of manufacturing facilities.
- Reduced capacity for post-market surveillance.
- Impact on the ability to investigate adverse events promptly.
Political Influence and its effect on FDA Decision Making:
Allegations of political influence on FDA decision-making during the Trump administration raised significant ethical concerns.
-
Examination of potential political pressures on the FDA approval process: Several instances raised questions about whether political considerations influenced drug approvals or regulatory decisions. These instances sparked debates about the agency's independence and its ability to remain unbiased in its assessment of new drugs and medical devices.
-
Long-term implications for biotech innovation and investor confidence: The perception of political influence can undermine investor confidence and potentially discourage long-term investment in biotech innovation. Concerns about regulatory unpredictability can hinder the development and launch of new therapies.
- Potential for chilling effects on research and development.
- Impact on investor decisions regarding biotech startups and established companies.
- Erosion of public trust in the FDA's regulatory processes.
The Lasting Legacy on Biotech Innovation:
The long-term consequences of the Trump administration's policies on the FDA are still unfolding.
-
Assessment of the long-term effects of Trump's FDA policies on the biotech landscape: The accelerated approval process, while potentially speeding up access to treatments, could have long-term repercussions if it compromises safety or leads to less effective therapies. The impact on innovation remains a subject of intense scrutiny and analysis.
-
Comparison with previous administrations’ approach: Comparing the Trump administration's approach to FDA regulation with those of previous administrations is crucial for understanding its unique impact and developing more effective regulatory frameworks for the future. Analyzing the differences in approval rates, review times, and regulatory priorities provides valuable insights.
Conclusion: Navigating the Future of Biotech After Trump's FDA
Trump's impact on the FDA and its regulatory processes has created a complex and uncertain future for the biotech industry. The legacy of accelerated approvals, budgetary changes, and perceived political influence continues to shape the industry's trajectory. Understanding this legacy is crucial for navigating the challenges ahead. To stay informed about FDA regulations and their impact on the future of biotech, subscribe to industry newsletters like [mention specific relevant newsletters or organizations]. Understanding the ongoing implications of Trump's legacy on the FDA is critical for shaping the responsible and effective development of life-saving treatments.

Featured Posts
-
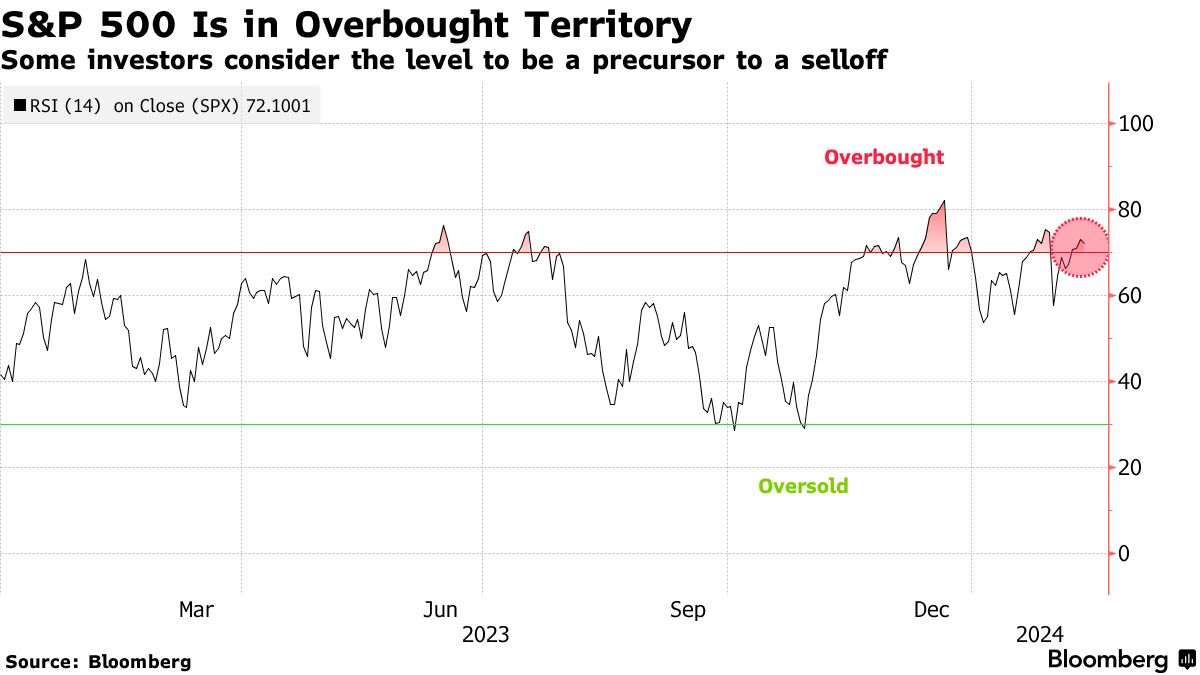 Understanding High Stock Market Valuations Insights From Bof A For Investors
Apr 23, 2025
Understanding High Stock Market Valuations Insights From Bof A For Investors
Apr 23, 2025 -
 Yankees Smash Team Record With 9 Home Runs Judges 3 Hrs Power Win
Apr 23, 2025
Yankees Smash Team Record With 9 Home Runs Judges 3 Hrs Power Win
Apr 23, 2025 -
 Cy Young Winners Dominant April Performance 9 Run Lead Strikeout Fury
Apr 23, 2025
Cy Young Winners Dominant April Performance 9 Run Lead Strikeout Fury
Apr 23, 2025 -
 La Vision De Pascal Boulanger Pour Le Developpement Immobilier En France Ou Autre Pays Selon Contexte
Apr 23, 2025
La Vision De Pascal Boulanger Pour Le Developpement Immobilier En France Ou Autre Pays Selon Contexte
Apr 23, 2025 -
 Breast Cancer Awareness Lessons From Tina Knowles Experience With Delayed Mammography
Apr 23, 2025
Breast Cancer Awareness Lessons From Tina Knowles Experience With Delayed Mammography
Apr 23, 2025
